Product Overview
Product Title
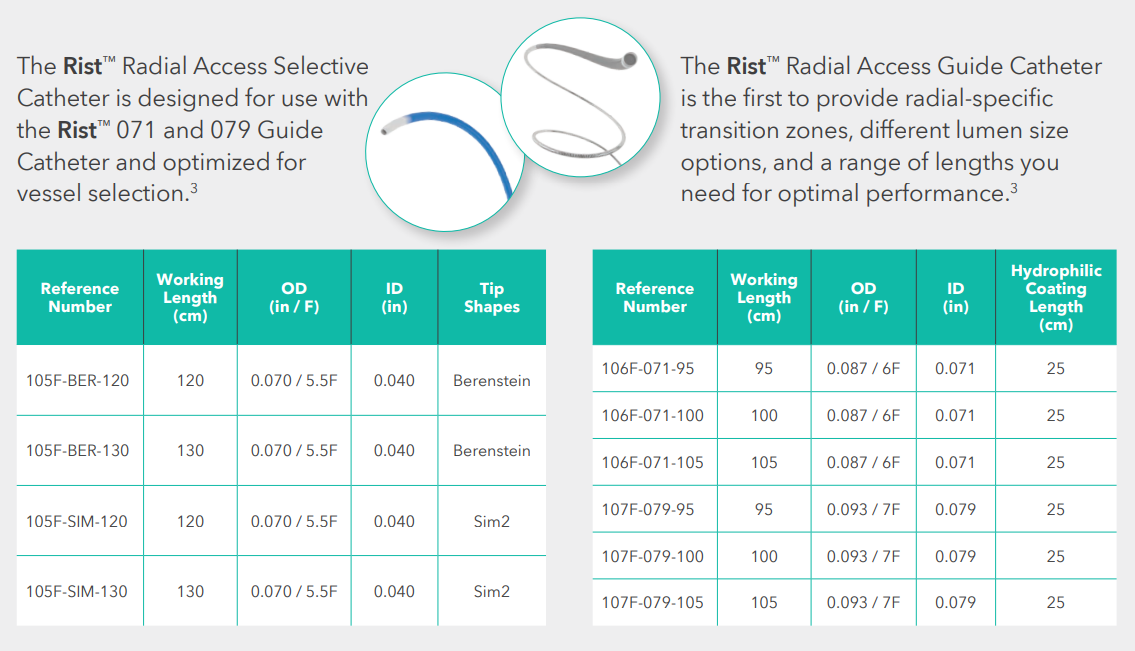
Medtronic Rist™ Radial Access Guide Catheter, 6F x 105 cm, REF 106F-071-105, Box of 1 –
EXPIRES: 2025-08-09
? Product Description
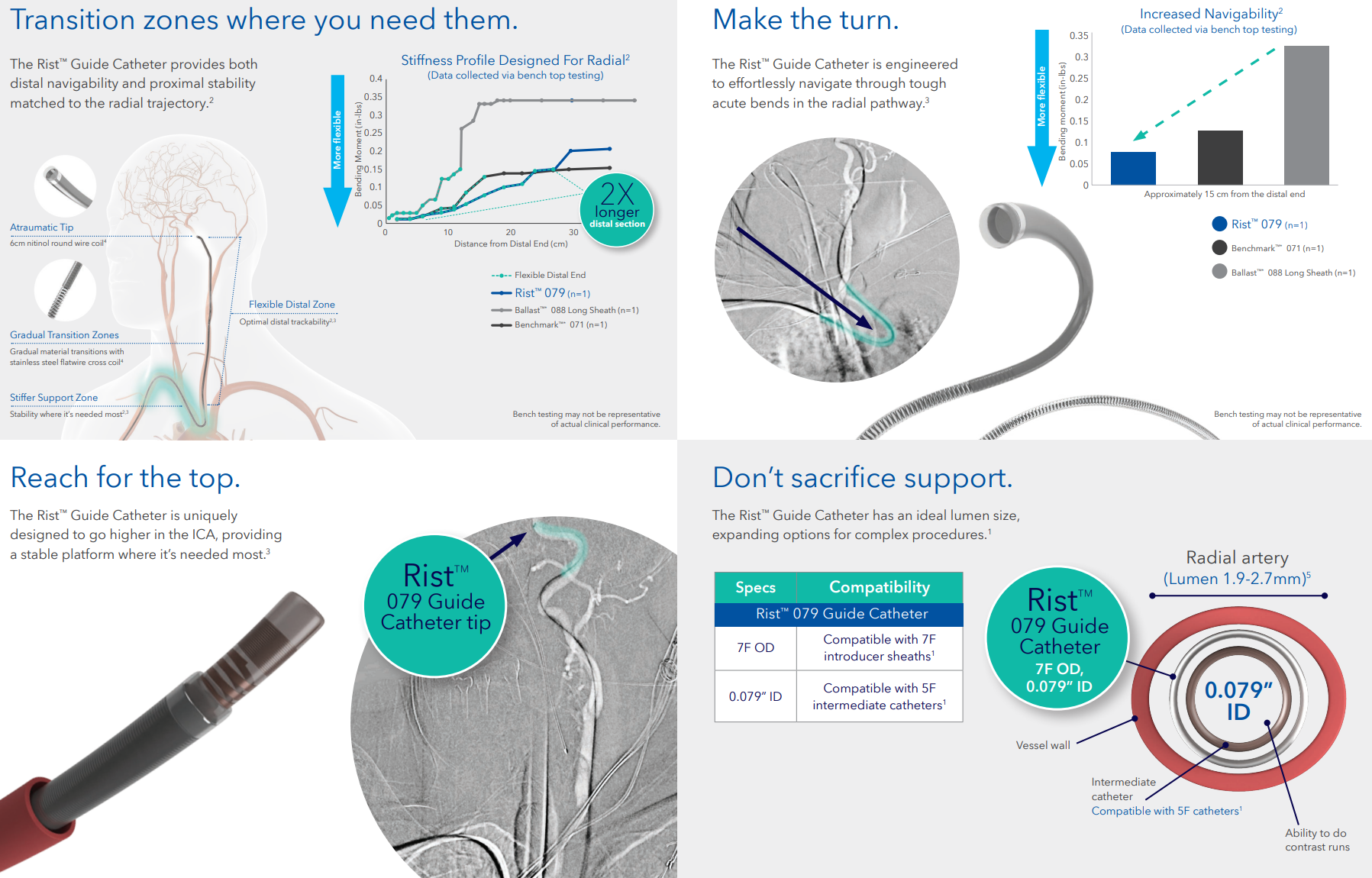
The Medtronic Rist™ Radial Access Guide Catheter is designed for the unique requirements of radial access in neurovascular procedures. With radial-specific transition zones, an optimized lumen diameter, and tailored length options, it ensures reliable support and smooth device delivery to target vessels.
The Rist™ system builds on Medtronic’s expertise in interventional access solutions, providing a dependable tool for peripheral, coronary, and neurovascular interventions.
? Key Features & Benefits
-
Radial-specific engineering for neurovascular interventions
-
6 French size (6F) suitable for a wide range of interventional devices
-
Length: 105 cm to reach distal target vessels with precision
-
Enhanced transition zones improve device trackability and support
-
Optimized lumen diameter for efficient delivery of interventional devices
-
Indicated for use in peripheral, coronary, and neurovasculature
-
Sterile, single-use, ready-to-deploy design
? Specifications
| Attribute | Details |
|---|---|
| Reference Number (REF) | 106F-071-105 |
| Brand | Medtronic |
| Product Type | Radial Access Guide Catheter |
| French Size | 6F |
| Length | 105 cm |
| Application | Peripheral, Coronary, Neurovascular Access |
| Sterility | Sterile |
| Quantity | Box of 1 |
| Expiration Date | 2025-08-09 |
Disclaimer: Expired products Not For Human Use. For Bench Testing and research use only. The item listed above is expired and may be used for educational, training, and non-clinical research purposes only. Any product information appearing below, including the product indication statement, pertains to an in date item only.